Structures and Boiling Points of Alkanes
Methane
Methane is the simplest alkane in the list of hydrocarbons. Its chemical formula is CH4 (Clayden, Greeves, and Warren 27). Its structure is made up of one carbon atom surrounded by four hydrogen atoms that are covalently bonded together.

As can be seen in the above methane structure, the three hydrogen atoms form the base of the structure at an angle of 109.5o while one hydrogen atom is stretched upwards from the central carbon atom at an angle of 108.70o is as shown below:

Its boiling point is −161.49 °C; or −258.68 °F; or 111.66 K
Ethane
Ethane follows methane in the homologous series of alkanes. Its structure is comprised of 2 carbon atoms each covalently bonded to three hydrogen atoms as shown in the diagram below:

Hence, the chemical formula of ethane is C2H6 while the boiling point is -128.2°F or -89°C.
Propane
The structure of propane is comprised of 3 carbon atoms covalently bonded to hydrogen atoms. Two of the carbon atoms are each bonded to three hydrogen atoms while one carbon atom is bonded to two hydrogen atoms as indicated in the structure below. The boiling point of propane is -43.6°F (-42°C).

n-Butane
There are two structural isomers of butane. The one indicated below is referred to as 2-methyl propane.
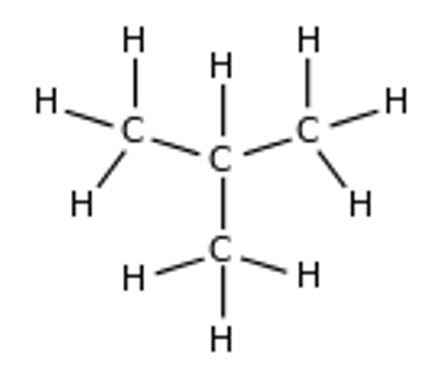
The structure of n-butane shows a methyl group covalently bonded to the middle carbon atom in the long propane chain. The compound records a boiling point of−13 to −9 °C (Clayden, Greeves, and Warren 103).
The other isomer of butane is the straight chain butane. Each of the isomer has a unique boiling point because they are different compounds altogether. The boiling point of a straight chain butane is-1°C.

n-Heptane
Heptane boils at 209.2°F (98.42°C). it is one of the alkanes with straight chains. Its molecular structure is H3C(CH2)5CH3 or C7H16 while the atomic structure is shown in the diagram below:

As can be seen in the figure above, n-Heptane has 7 carbon atoms all covalently bonded to each other and the respective hydrogen atoms (Clayden, Greeves, and Warren 73). From the 3-dimensional structure of n-heptane, it is evident that each of the hydrogen atoms is symmetrically bonded to carbon atoms with the latter atoms at the center.

Heptane has nine different isomers
n-Hexane
Hexane has 5 isomers with different Boling Points (BP). These are:
- 2,3-dimethylbutane- BP of 57.9 to 58.3 °C
- 2,2-dimethylbutane-BP of 50°C
- 3-methylpentane6-BP of 62.9 to 63.7 °C
- 2-methylpentane-BP of 60°C
- Hexane-BP of 68°C
BP Trends
The straight chain hexane has the highest boiling point because the molecule occupies the largest space possible and therefore more heat energy is required to break the bonds. The highly symmetrical isomers of hexane have the least boiling points because they are highly compact. Any little available heat energy can reach all the bonds and break them evenly.
n-Hexane also falls within the homologous series of alkanes. Its molecular formula is C6H14. It implies that the structure of n-hexane has six carbon atoms and 14 hydrogen atoms all covalently bonded to each other.

Boiling point trend
The following are some of the important points that can be derived from the structure and boiling points of organic compounds:
- Organic compounds with lower carbon and hydrogen atoms have a lower boiling points because there are fewer covalent bonds (intermolecular forces) to be broken at any given time. In other words, the higher the numbers of carbon and atom atoms, the higher the boiling points. For example, butane has a lower boiling point than any of the isomers of hexane since it has fewer bonds to be broken.
- Lower boiling points are recorded among branched chain organic compounds (Clayden, Greeves, and Warren 65). Hence, all the isomers drawn from the parent organic compound have lower boiling points since the molecule becomes more compact owing to the branched chain. As a result, the surface area is reduced and consequently minimizes the strength of intermolecular forces. Hence, any branched chain alkane is broken quite easily during the heating process.
References
Clayden, Jonathan, Nick Greeves, and Stuart Warren. Organic Chemistry, New York: Oxford University Press, 2012. Print.
